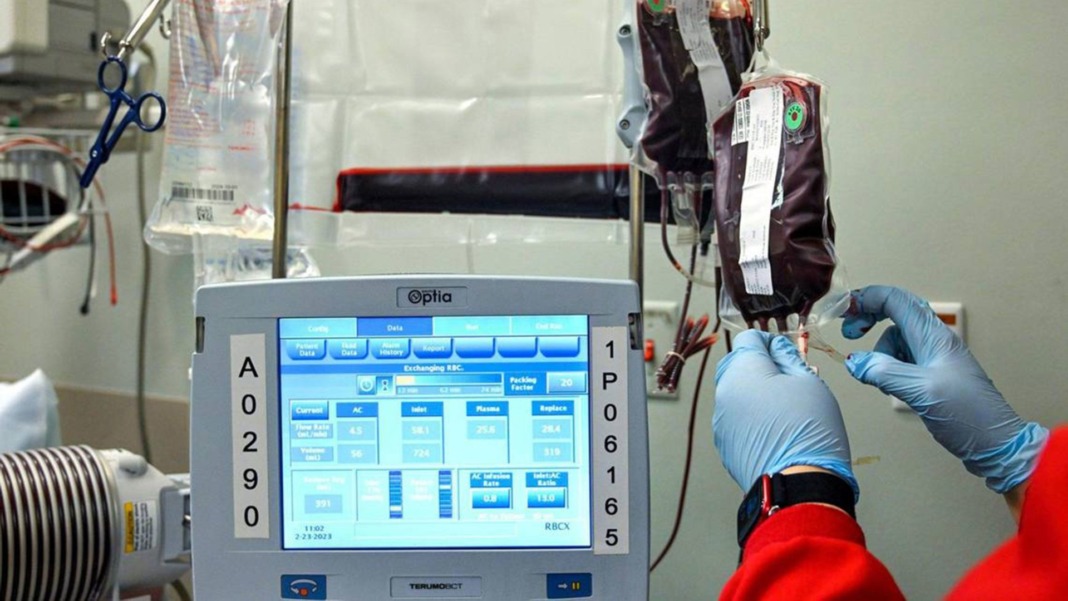
In an unprecedented turn of events, a potential game-changer in the treatment of sickle cell disease is currently under scrutiny by the US Food and Drug Administration (FDA). The therapy in question, known as exa-cel, utilizes the revolutionary CRISPR gene editing technology and has the potential to transform the lives of individuals grappling with the excruciating and often fatal effects of sickle cell disease.
At the heart of this groundbreaking discussion is Dr. Lakiea Bailey, the 45-year-old executive director of the Sickle Cell Consortium. Diagnosed with sickle cell disease at the tender age of 3, Dr. Bailey has faced a lifetime of health challenges, from heart problems to hip replacements, all while enduring chronic pain. Yet, she stands as a beacon of hope for the sickle cell community, testifying before the FDA’s independent advisory committee on Tuesday about the transformative potential of exa-cel.
“Hope is on the horizon, and we are looking toward this hope for a change in the lives that we are living of excruciating pain,” Dr. Bailey passionately declared during the FDA committee meeting.
Sickle cell disease is a rare genetic disorder that disproportionately affects African Americans. In the United States alone, approximately 100,000 people are diagnosed with the condition, and of those, 20,000 are classified as having severe disease. Historically, treatment options have been limited, with stem cell or bone marrow transplants serving as the primary approaches. However, the scarcity of compatible donors and the inherent risks associated with these procedures have posed significant challenges.
Enter exa-cel, a potential paradigm shift in sickle cell treatment developed collaboratively by Boston-based Vertex Pharmaceuticals and CRISPR Therapeutics. Unlike traditional transplants, exa-cel harnesses the power of the patient’s own stem cells, which are modified using CRISPR technology to rectify the genetic anomalies responsible for sickle cell disease. These genetically corrected stem cells are then reintroduced to the patient through a one-time infusion.
Dr. Stephanie Krogmeier, vice president for global regulatory affairs with Vertex Pharmaceuticals, presented promising findings from company studies to the FDA committee. The treatment demonstrated safety, with 39 out of 40 individuals showing no signs of vaso-occlusive crises, a painful condition where misshapen red blood cells obstruct normal circulation.
Despite the groundbreaking potential, the FDA sought the advice of its independent panel due to the unprecedented use of CRISPR technology. Dr. Fyodor Urnov, a professor at the University of California, Berkeley, reassured the committee, highlighting 30 years of CRISPR research and asserting that the technology is ready for mainstream applications.
While the data presented was generally well-received, the FDA’s presentation raised concerns about the lack of confirmatory testing and the study’s relatively small size. However, several panel experts expressed confidence in the reasonability of the data, with Dr. Scot Wolfe, a temporary voting member of the committee, commending the company’s commitment to monitoring patients for 15 years.
As the discussions unfold, Dr. Daniel Bauer, principal investigator and staff physician at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, addressed concerns about potential off-target effects of CRISPR technology. He emphasized the relative smallness of the risk and urged a balanced approach, acknowledging uncertainties while recognizing the urgency of advancing therapies for individuals with sickle cell disease.
The FDA is expected to make a pivotal decision on the approval of exa-cel by December 8, marking a potential milestone in the treatment of sickle cell disease. This decision not only holds implications for the future of sickle cell patients but also marks a crucial step forward in the broader landscape of genetic editing technologies and their applications in medical treatments. As the scientific community awaits the FDA’s decision, the potential approval of exa-cel stands as a beacon of hope for those who have long endured the hardships of sickle cell disease.