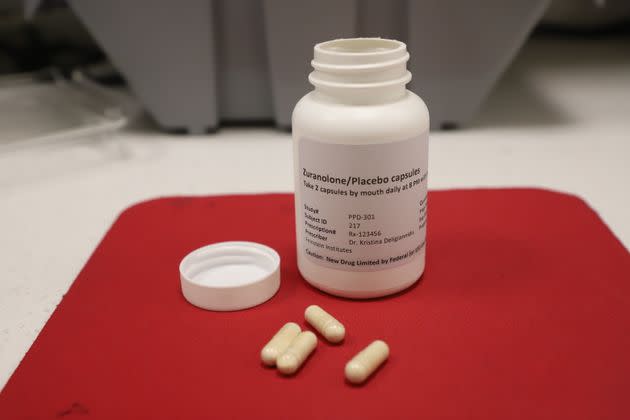
In a monumental stride towards better maternal mental health, the US Food and Drug Administration (FDA) has granted its approval for the groundbreaking medication zuranolone to be used in the treatment of postpartum depression. This historic decision marks the first time an FDA-approved oral pill has been designed explicitly to combat the complex and often debilitating condition affecting approximately 1 in 7 new mothers after childbirth.
The announcement, made on a Friday, revealed that the newly approved treatment, to be marketed under the brand name Zurzuvae, will be administered as a once-daily pill taken over a 14-day course. The severity of postpartum depression cannot be understated, encompassing emotions such as overwhelming sadness, guilt, and an often crippling sense of worthlessness. In extreme cases, these feelings can escalate to include thoughts of self-harm or harm to the newborn. Dr. Tiffany R. Farchione, Director of the Division of Psychiatry in the FDA’s Center for Drug Evaluation and Research, noted the pivotal significance of this approval in providing women with an invaluable oral treatment option to manage these intense and sometimes life-threatening emotions.
However, the FDA’s decision came with a caveat in the form of a boxed warning added to the drug’s labeling. This warning highlights the potential impact of zuranolone on activities that require heightened attention and coordination, such as driving. The FDA advises users of zuranolone to abstain from operating heavy machinery or driving for at least 12 hours after taking the medication, thereby mitigating any potential risks associated with its use.
Zuranolone, while holding the promise of easing the burden of postpartum depression, does come with its share of potential side effects. These commonly include drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis (common cold), and urinary tract infections. Of more significant concern is the potential for zuranolone to induce suicidal thoughts and behavior, along with possible harm to the developing fetus. To counteract these risks, the FDA recommends that women employ effective contraception while using zuranolone and for a week following its completion.
The implications of postpartum depression extend far beyond the individual affected. With maternal deaths from suicide accounting for a substantial 20% of all postpartum fatalities, it becomes increasingly evident that this condition demands effective treatment. The United States sees over 400,000 births annually to mothers grappling with depression, with untreated cases potentially lasting for months or even years, as outlined by the National Institute of Mental Health.
The development of zuranolone is credited to the collaborative efforts of drugmakers Biogen and Sage Therapeutics. Zuranolone represents the second FDA-approved medication to address postpartum depression, the first being Zulresso. The key differentiator between the two lies in their administration methods. While Zulresso requires a 60-hour intravenous drip of the drug brexanolone within a healthcare setting, zuranolone’s oral intake offers a more accessible and convenient approach to treatment.
Both zuranolone and Zulresso derive from allopregnanolone, a natural substance within the body involved in mood regulation. These medications function by restoring allopregnanolone levels, thereby providing relief from the symptoms of postpartum depression. The FDA’s approval of zuranolone stands as a monumental stride in the field of maternal mental health, granting women access to a swift-acting treatment that can significantly impact their overall well-being.
While the approval of zuranolone is undoubtedly a cause for celebration, it does raise certain concerns within the mental health community. Dr. Catherine Monk, a professor at Columbia University Vagelos College of Physicians and Surgeons, acknowledges the potential for misuse due to the medication’s original trials being primarily focused on severe cases of postpartum depression. This has led to concerns about overprescribing zuranolone for milder forms of the condition. Mental health experts emphasize the importance of considering psychotherapy and behavioral interventions in conjunction with medication.
The FDA’s approval of zuranolone is a monumental step forward in the realm of maternal mental health treatment. While the medication offers a promising solution, it remains crucial to address the social determinants of health and recognize the diverse needs of patients for a holistic approach to care in this domain. As we mark this significant achievement, it’s imperative to remember that addressing postpartum depression requires a multifaceted approach that encompasses medical, psychological, and societal factors to truly make a lasting impact on the lives of new mothers and their families.