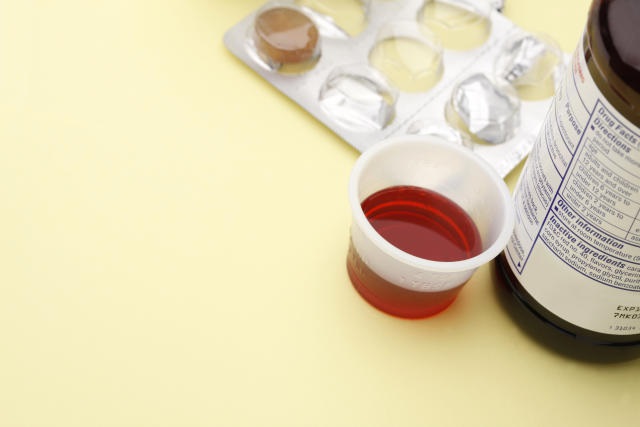
Washington, D.C. – In response to hundreds of overseas deaths attributed to contaminated cough syrups, the U.S. Food and Drug Administration (FDA) is intensifying efforts to ensure the safety testing practices of healthcare products in the United States. A recent review of regulatory alerts by Reuters has revealed that the FDA has reprimanded at least 28 companies in 2023 for inadequate testing of ingredients used in over-the-counter drugs and consumer products, specifically concerning toxins ethylene glycol (EG) and diethylene glycol (DEG).
The manufacturers under scrutiny are a mix of U.S.-based companies and exporters from around the world, including India, South Korea, Switzerland, Canada, and Egypt. The FDA’s actions signal a significant increase in vigilance compared to previous years, as more companies have been flagged for failing to test raw materials susceptible to EG and DEG contamination this year than in the previous five years combined.
Notably, the FDA has clarified that there is no evidence of DEG and EG-contaminated products entering the U.S. supply chain. Peter Lindsay, a Washington, D.C.-based lawyer specializing in FDA regulation and compliance, emphasized that the agency is raising its standards by requiring manufacturers to test individual containers of ingredients rather than merely sampling raw materials.
The urgency for enhanced oversight arises from a global crisis involving contaminated cough syrups, predominantly manufactured in India and Indonesia, which have been linked to over 300 deaths, primarily children, worldwide. These tainted medicines contained elevated levels of DEG and EG, resulting in acute kidney injuries and fatalities.
This crisis has triggered criminal investigations, lawsuits, and intensified regulatory scrutiny worldwide. Recent reports by Reuters have revealed that some Indian drugmakers involved in the production of these cough syrups could not verify the purchase of pharmaceutical-grade ingredients or the testing of their medicines for these harmful toxins.
In the United States, over 100 people, mostly children, succumbed to DEG poisoning in the 1930s, leading to the enactment of laws significantly bolstering the FDA’s regulatory authority over drugs. However, explicit rules for testing high-risk ingredients like propylene glycol (PG) and sorbitol solution for EG and DEG contamination were only established in May 2023.
Previously issued guidance in 2007 recommended specific tests on glycerin, a common ingredient in over-the-counter drugs and consumer goods, to prevent the distribution of DEG-contaminated products. This guidance has now been extended to require similar scrutiny of PG and other high-risk components for DEG and EG.
The FDA’s warning letters provide manufacturers with an opportunity to rectify quality control issues, with the threat of penalties looming for non-compliance. Manufacturers who do not improve their testing practices risk having their exports or imports blocked and their new drug applications rejected.
Alarmingly, eleven of the companies cited by the FDA this year marketed products susceptible to DEG and EG poisoning to children, including diarrhea and pink eye medicines, toothpaste, and sunscreen.
One of the manufacturers called out by the FDA is Lex, a Florida-based contract manufacturer of cough and cold medicines that can be used by children. Lex has pledged to address the identified shortcomings and conduct all required tests for impurities whenever they obtain ingredients susceptible to EG and DEG contamination.
Fourteen foreign manufacturers, including South Korea’s LCC, which produces Oriox mouthwash, and India-based toothpaste manufacturers Suhan Aerosol and Orchid Lifesciences, were placed on import alert lists for failing to prove sufficient quality control. While LCC is responding to the FDA, Suhan and Orchid have asserted that EG and DEG were not found in their products.
Additionally, four of the 14 companies on the import alert list were cited for failing to respond to record requests, including Daxal Therapeutics and Skyline Herbals from India, and South Korea’s KM Pharmaceutical and Sangleaf Pharma.
Moreover, the FDA has warned 13 U.S. manufacturers of consumer products, such as earwax removers, nasal spray, hand soap, and shampoo, of possible seizures and injunctions. These companies were reprimanded for failing to conduct required contamination checks and, in some cases, relying on suppliers’ certificates of analysis for ingredient purity.
Greg Landry, an expert in pharmacology and toxicology at the Massachusetts College of Pharmacy & Health Sciences, acknowledged the challenge of policing every consumer product but commended the FDA’s swift and robust response when problems are identified. As the FDA strengthens its oversight, it aims to ensure that healthcare products entering the market are safe and free from harmful contaminants, prioritizing the health and well-being of consumers.