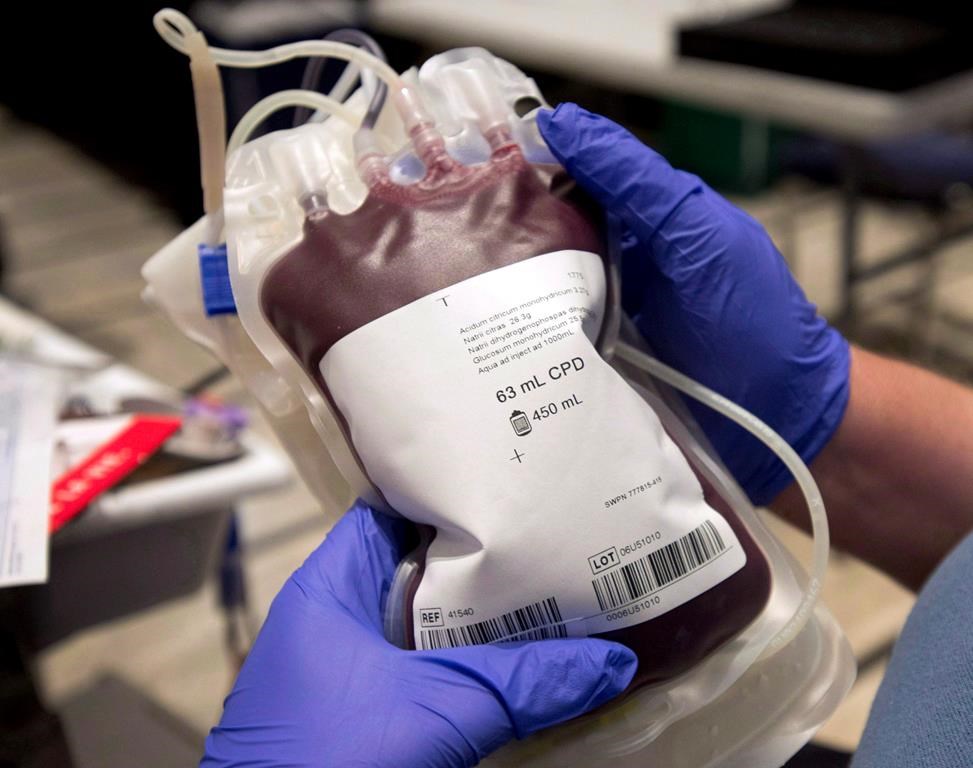
An Alberta couple, Carly and Ben Seligman, have voiced serious concerns about a potential contamination at the Canadian Cord Blood bioRepository (CCBR) in west Edmonton. The Seligmans, along with many others, have entrusted the facility with their children’s umbilical cord blood, expecting safe and reliable storage. However, the recent revelations have raised significant doubt about the facility’s compliance with health safety standards.
Health Canada has issued a public warning regarding the CCBR, stating that it remains in violation of health safety standards. Some of the issues reported include inadequate measures to monitor temperature, humidity, and possible contamination during processing, testing, and storage. In light of these serious concerns, the clinic has been prohibited from accepting new specimens until it submits and implements a corrective action plan.
Despite these damning findings, the founder of CCBR, Dr. John Akabutu, maintains that the facility is in full compliance with the necessary standards. He claims that the Health Canada inspectors were misinformed about the regulations, leading to the controversy. However, the federal Food and Drugs Act, which regulates all cord blood banks, does not subject private banks like CCBR to routine inspections, raising questions about the facility’s actual compliance status.
In the wake of this crisis, the Seligmans are demanding more transparency and accountability from the operator. They, along with other parents, are questioning the viability and safety of their children’s cord blood, expressing a deep-seated breach of trust. Health Canada has warned that stored specimens should be tested before use in medical treatments to ensure their safety and viability.
The controversy has also sparked a broader conversation about the value of cord blood and the promotion of yet-to-be-developed stem cell therapies. As private cord blood banks are known to inflate the likelihood of the need for such specimens, the Seligmans have encouraged parents to consider donating to public cord banks instead.
Health Canada is continuing to monitor the CCBR closely. If the facility fails to address the concerns adequately, additional enforcement actions may be taken. This includes the potential for the facility to be barred from operating altogether. In the meantime, customers are advised to contact the company to understand their options regarding the stored specimens.
This incident has underscored the importance of stringent regulation and oversight in the healthcare sector, especially concerning facilities that deal with critical biological materials. Cord blood banking is a rapidly growing industry, driven by promises of potential life-saving treatments using stem cells derived from cord blood. However, the efficacy and safety of these treatments depend heavily on the integrity and quality of the stored specimens.
Furthermore, the controversy surrounding CCBR highlights the challenges of balancing innovation and consumer protection in emerging fields such as regenerative medicine. While advancements in stem cell research hold tremendous promise for treating a wide range of medical conditions, they also present ethical and regulatory challenges that must be addressed to ensure patient safety and trust.
As the story continues to unfold, it serves as a stark reminder of the need for transparency, accountability, and robust regulatory frameworks in healthcare. Ultimately, the well-being of patients and the integrity of medical treatments should always take precedence over commercial interests. As consumers, it is crucial to stay informed and advocate for measures that uphold the highest standards of safety and ethics in healthcare practices.